Mahnaz Azimzadeh Sani Kristina Tschulik in Frontiers of Nanoscience 2021. Having few or no active chemical properties.
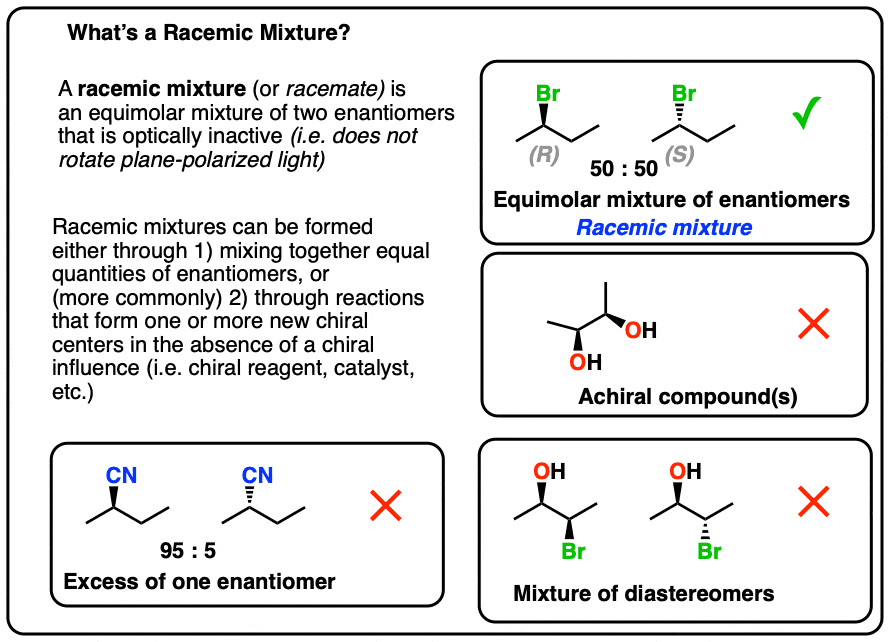
What S A Racemic Mixture Master Organic Chemistry
Basically physical properties are those which you can observe and measure without changing the chemical identity of your sample.
. Zinc Zn is a silver gray element that can be ground into a powder. In this type of impact electrochemically inactive particles of nano- or micrometer size are detected indirectly by analysis of the decrease in the steady-state current of a solution-phase redox couple indicator at an electrode. Halogen A nonmetallic element that combines readily with most metals.
Updated on January 24 2020. The scale used to classify the strength of acid or basic solutions. The first layer of the Earths atmosphere touching Earths surface.
As one might suspect the inactive ingredients are far from inactive either when tested alone or when combined with the active ingredients. An indifferent chemical in a reaction. EPA-approved product that is at once a viricide bactericide sanitizer insecticide deodorant germicide-disinfectant mildewcide fungicide bacteriostatic and fungistatic.
The study of the make-up structure and properties of matter. A chemical property is a characteristic or behavior of a substance that may be observed when it undergoes a chemical change or reaction. Iron for example combines with oxygen in the presence of water to form rust.
The heavy radioactive metals with atomic numbers 90 through 103. Iners unskillful sluggish fr. Chemical properties are seen either during or following a reaction since the arrangement of atoms within a sample must be disrupted for the property to be investigated.
As the sub-shells of noble gases is fully filled and their octet is complete. The chemical property of a substance is determined by the loss or gain of electrons. Chromium does not oxidize Figure 2.
Examples of chemical properties include flammability toxicity acidity reactivity many types and heat of combustion. Radioactivity - The emission of radiation from an atom with an unstable nucleus is a chemical property. Having only a limited ability to react chemically.
3 After filtering the NCI database we performed virtual-docking screening against the corresponding optimized 3D chemical compound library using either the inactive or active CB2 model respectively thus we. The parameters used for the generation of the pharmacophore models and UNITY search in SYBYL were described in our previous publication. Chemical descriptors encode the physicochemical and structural properties of small molecules and they are at the core of chemoinformatics.
Fluorine is the most electronegative element on the periodic table and thus is the most chemically reactive. It is highly effective at killing bed bugs. The change of one type of matter into another type or the inability to change is a chemical property.
Physical properties are used to describe matter and make observations about it. Examples of physical properties include color shape position volume and boiling point. A chemical reaction is a process that occurs when one or more substances are changed into one or more new substances.
In chemistry when compounds interact it is not always a simple linear one to one relationship. Iron for example combines with oxygen in the presence of water to form rust. Nitroglycerin is very dangerous because it explodes.
Unable to move or resist motion. Sterifab is the only US. In general the least.
Lanthanide Series The rare-earth elements with atomic numbers 58 through 71. Elements that are near fluorine also have high electronegativity values and are also highly chemically active. Whats more Sterifab can be sprayed on everything except people animals and cooking utensils.
She was fat and inert. The first time I mentioned them to my internist a. Elements that are highly electronegative are highly reactive while elements that have low electronegativity are less reactive.
Excipients are officially designated and therefore often dismissed as inactive ingredients. A mind grown torpid in old age. Chromium does not oxidize.
Denoting a drug or agent having no pharmacologic or therapeutic action. Chromium does not oxidize. Only the active ingredients are tested singly and never with the entire chemical cocktail that is the product itself.
Having few or no active chemical properties. A chemical change is also called a chemical reaction. The change of one type of matter into another type or the inability to change is a chemical property.
Examples of oxidation include the way an apple turns brown after it has been cut the way a penny turns green and the way a fender on a car can become rusty. On the periodic table of elements the elements that have no stable isotopes are. Mendeleev Dimitri A Russian chemist who devised the periodic table.
Upon landing of a particle the electroactive surface. Tetracycline antibiotics have been a mainstay of infection control both in the clinic and in agriculture for over 50 years 4They have well-established chemical properties and. The change of one type of matter into another type or the inability to change is a chemical property.
Examples of chemical properties include flammability toxicity acidity reactivity many types and heat of combustion. The broad release of bioactivity data has. In-ert 1.
Iron for example combines with oxygen in the presence of water to form rust. Devoid of active chemical properties as the inert gases. Nitroglycerin is very dangerous because it explodes.
If zinc is mixed at room temperature with powdered sulfur S a bright yellow element the result will. Examples of chemical properties include flammability toxicity acidity reactivity many types and heat of combustion.
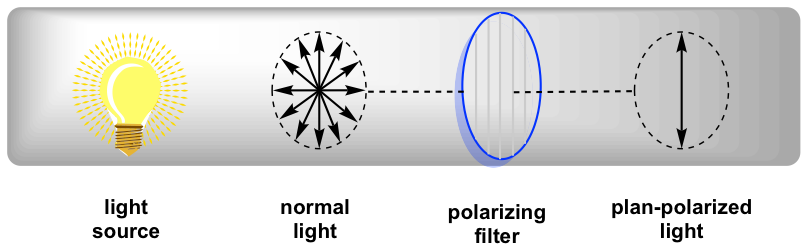
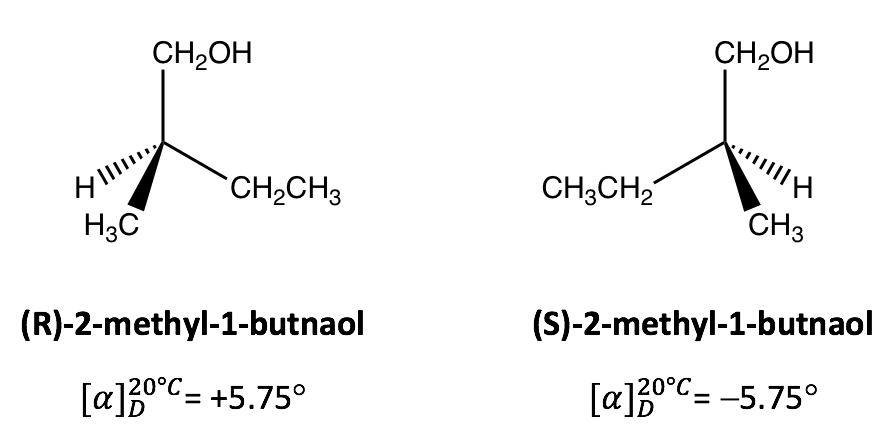
5 4 Optical Activity Organic Chemistry I

5 4 Optical Activity Organic Chemistry I
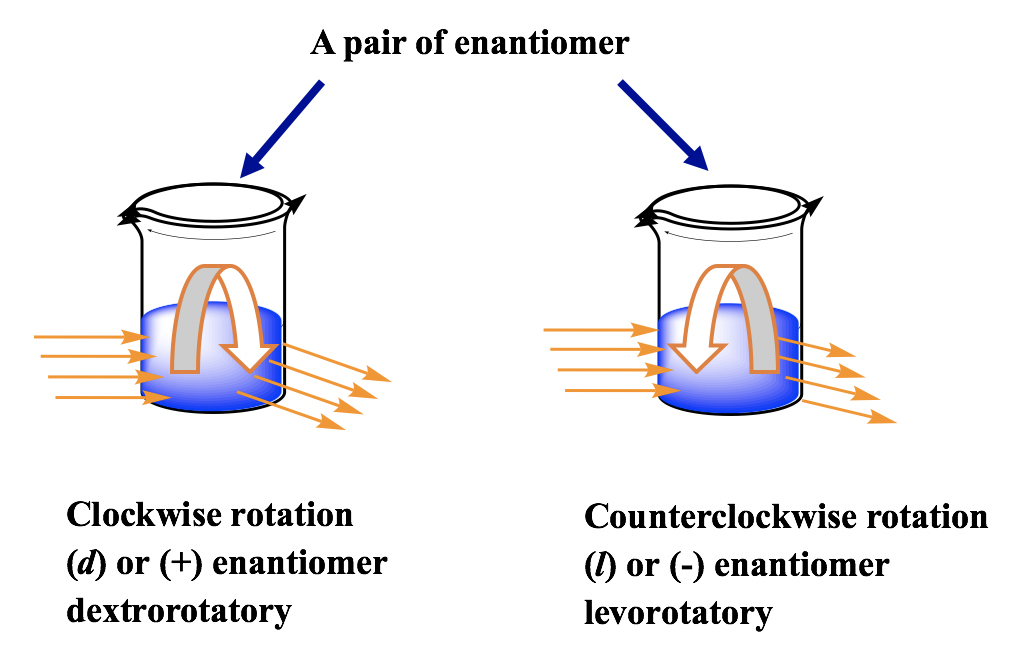
5 4 Optical Activity Organic Chemistry I
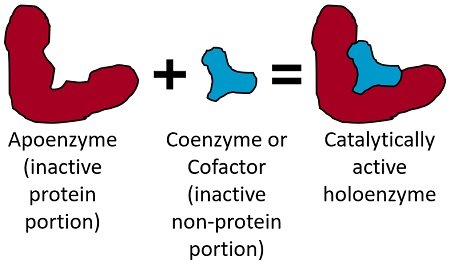
General Properties Of Enzyme Physical Chemical Properties Biology Reader

0 Comments